Adults with brain metastases (BM) have the most prevalent intracranial tumours. Between 20% and 40% of cancer patients acquire brain metastases (BM), for which a number of treatment options, including surgery and radiation, are available. Stereotactic radiosurgery (SRS) has been demonstrated as an effective treatment option for brain metastases (BM). Stereotactic radiosurgery (SRS) can be used in conjunction with or in place of previous surgical resection (Sx) or whole-brain radiation treatment (WBRT).
Stereotactic radiosurgery (SRS) is a high-precision technology that delivers a considerable dosage of radiation to a localised portion of an organ while minimising radiation to surrounding healthy tissue. Dr. Lars Leksell described conventional radiosurgery in the form of the Gamma Knife (Elekta, Stockholm, Sweden) in 1951. In order to target a specific region in the brain, this approach required the deployment of a hard head frame to immobilise the patient. Although this intrusive technique is highly accurate, it has a number of downsides for the patient, including discomfort and anxiety. Furthermore, the stiff head frame necessitates the presence of a neurosurgeon during frame installation.
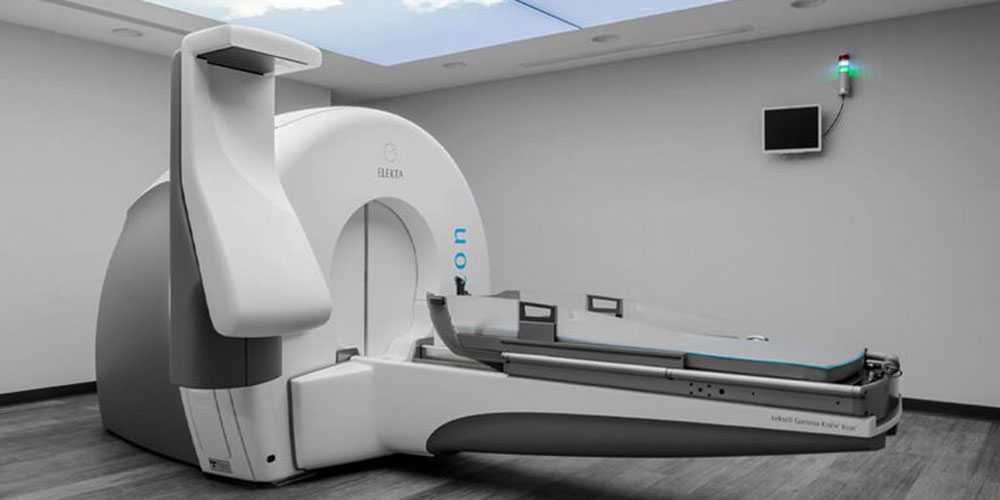
There are currently a number of frameless SRS systems available that use linear particle accelerator (LINAC) technology and do not require the implantation of a surgical hard frame while enabling improved conformance to odd-shaped lesions and sparing of eloquent areas.
A few days before the intervention, patients were immobilised using a frameless system and an individualised mask moulded with a thermoplastic pellet. To improve stiffness, a personalised intra-oral thermoplastic component was inserted. The time it took to make these masks ranged between 30 and 45 minutes.
Following the mask moulding, a contrast computed tomography (CT) scan of the brain with a reconstructed slice thickness of 1 to 1.5 mm was conducted. The scans were then combined with gadolinium-enhanced magnetic resonance imaging (MRI) of the brain, which had previously been done. A radiation oncologist then contoured the lesion, and a margin of 1 to 3 mm was added to the gross tumour volume (GTV) to get the planned targeted volume (PTV). Before commencing dosimetry planning, a second radiation oncologist reviewed the contour of the target lesions and the organs at risk.
The system was Elekta Synergy-S®. An on-board cone-beam CT was used to confirm the patient's placement. For adjustments, a HexaPODTM (Elekta) table was also employed.
This enables repositioning along all axes, including rotations.
Patients might get either a single dose of radiation or a divided treatment. The planned targeted volume PTV was given a dosage ranging from 12 to 24 Gy, with a 15 Gy median. The ultimate radiation dosage was determined by the location and maximum diameter of the tumour. Furthermore, the volume of tissue receiving more than 10 Gy (V10) or 12 Gy (V12) was taken into account in the final radiation dose since it is an important predictor of radionecrosis (RN).
Our institution's treatment of brain metastases (BM) with linear particle accelerator (LINAC)-based frameless stereotactic radiosurgery (SRS) showed an overall and progression-free survival equivalent to the literature for frameless stereotactic radiosurgery (SRS) and traditional frame-based stereotactic radiosurgery (SRS) while being less intrusive and more pleasant for the patient. According to our findings, frameless stereotactic radiosurgery (SRS) using linear particle accelerator (LINAC) technology appears to be safe for brain metastases (BM) therapy with low incidences of radiation necrosis.
Frameless Stereotactic Radiosurgery
Laser Disc Decompression
Radiofrequency Ablation for Trigeminal Neuralgia
Radiofrequency Ablation for Back Pain & Neck Pain
Minimal Invasive Spine Surgery
Spine Stabilization
Cranial Micro Neurosurgery
Cranio Spinal Trauma
Endoscopic Neurosurgery